[ad_1]
Written by Venu Malarapu

Despite challenges in developing treatments for rare diseases, there is hope for the fewer than 200,000 people around the world living with one (or more) of the 7,000 identified diseases, and industry is is steadily increasing its research efforts. According to a study by the Tufts Center for Drug Development and Research, 34% of drugs in research and development (as of 2020) target rare diseases. This is a significant increase from 9% in 2010. Additionally, 31 of the 53 new drugs approved by the FDA’s CDER in 2020 were for rare diseases or conditions.
One of the catalysts for increased attention to rare diseases was the passage of the Orphan Drug Act (ODA) in 1983. As the graph below shows, the number of orphan drug designations has increased sevenfold over the last 10 years (2013-2022).
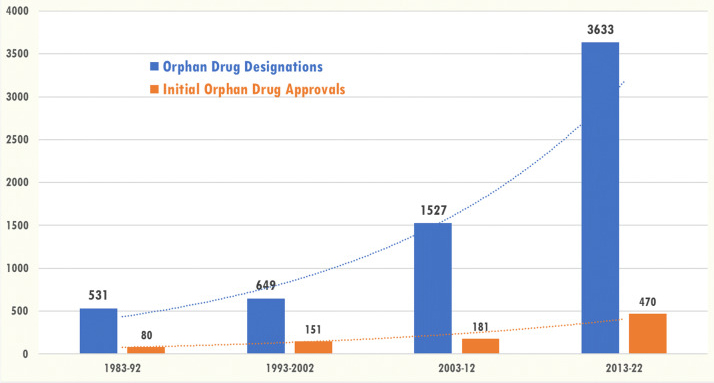
Total number of orphan drug designations (n = 6,340) and number of first orphan drug approvals (n = 882) during the decade 1983-2022
Used under the Creative Commons Attribution 4.0 International License. Source: Comprehensive study of rare diseases and conditions eligible for orphan drug designation and approval over 40 years of the Orphan Drug Act – PMC (nih.gov).
Challenges in clinical research on rare diseases
As mentioned in the section above, finding and recruiting patients for clinical trials is difficult given the small number of patients affected by rare diseases. This often results in small studies with inconclusive results.
Additionally, many rare diseases are poorly understood, making it difficult and time-consuming to develop effective treatments and conduct research into their safety and effectiveness. It costs money. Incentives provided through ODA, such as orphan drug designation and a 25% tax credit on research and development costs, certainly help.
Another challenge with rare diseases is that symptoms can vary from patient to patient, even with the same rare disease. This makes it difficult to measure the effectiveness of treatment.
Given that patients with rare diseases are so rare, researchers need to protect patient safety and well-being. Therefore, ethical considerations become paramount and decisions such as placing a patient on a placebo or control group may not be possible.
Finally, there is the financial aspect. Some diseases that are more prevalent among large populations provide incentives for pharmaceutical companies to invest in clinical research. These companies are likely to benefit financially if successful treatments are discovered. This may not be the case for rare diseases, given that the population affected by rare diseases is small and fewer people can afford the treatments.
Using RWD can reduce challenges in rare disease research
However, there are several ways to address some of the above challenges, including the use of real-world data (RWD) supported by machine learning (ML). RWD helps:
- fill data gaps: Supplement small clinical trials and identify potential participants.
- Improving understanding of disease: Track disease progression, identify risk factors, and inform personalized medicine.
- Trial optimization: It helps you adjust endpoints, calculate sample size, and design better trials.
- Safety and efficacy monitoring: Track long-term outcomes and uncover rare drug interactions and adverse events.
- Support regulatory decisions: Helps approve new treatments and update treatment guidelines.
However, it is important to recognize the limitations of RWD in rare diseases such as:
- Data quality issues exist, including missing data due to incomplete medical records, record discrepancies due to variations in data collection, and measurement errors in diagnostic coding.
- Potential biases include selection bias, where patients included in the RQD may not be representative of the entire rare disease population. Confounding biases such as socio-economic status and comorbidities. Information bias refers to missing or inaccurate data that lead to biased conclusions.
- Causal inference is challenged by the lack of controlled comparisons used in randomized trials. Temporal disruption, as time-dependent factors can influence disease progression and treatment decisions. In the case of reverse causation, the disease itself may influence the choice of treatment, creating a reverse causal pathway and complicating the analysis.
These must be handled carefully. Nevertheless, with responsible data collection, analysis, and interpretation, RWD can be a powerful tool to overcome data challenges and accelerate advances in rare disease research and clinical care.
Where data challenges remain in rare diseases
Data challenges in rare diseases are significant and complex, posing major hurdles to therapeutic research and development. Some of the main ones are listed below.
- Lack of data
- A small number of patients results in insufficient data being collected for meaningful analysis. Small datasets lack statistical power and are difficult to use to draw definitive conclusions.
- Patient data is often spread across different healthcare institutions, patient registries, and studies. This results in a lack of centralization and standardization, making it difficult to integrate and analyze patient data to make informed decisions.
- poor quality
- Differences in medical formats, diagnostic criteria, and collection methods all lead to data inconsistency and heterogeneity. (This is also a problem for clinical research in non-rare diseases.) This makes it difficult to compare data across different sources, such as local laboratories, facilities, and data collection systems.
- Missing data across different organizations’ medical records for the same patient complicates aggregation and analysis.
- Access and share
- Privacy concerns are a perennial issue, even more so when it comes to rare diseases. Sharing data across institutions and countries is difficult, which limits collaboration and research progress.
- Lack of interoperability due to differences in data formats and lack of standardized data models creates barriers to aggregating and analyzing data from different sources.
- technology
- The lack of robust data storage, management, and analysis tools limits the effective use of already limited data in rare diseases.
- Small and heterogeneous datasets in rare diseases pose challenges in applying advanced data analysis techniques, including machine learning and other novel approaches.
![]()
Apart from these, especially in rare diseases, there are always ethical aspects to ensure the protection of vulnerable patient populations, such as obtaining consent and ultimately sharing the benefits of research with patients. .
Integrated clinical data cloud platform helps overcome rare disease data challenges
An integrated clinical data cloud platform is a data and analytics platform that includes a data hub for aggregating clinical data from multiple data sources in multiple formats, supports data transformation and standardization, supports computing and analytics, It has applications to use the data for management. and decision making. These range from off-the-shelf platforms to in-house built applications, but their use is increasing as the industry realizes the benefits of such technology investments. The use of such platforms for rare diseases has tremendous potential to overcome many of the data challenges mentioned above by:
1. Data aggregation
It provides the ability to pool data from across institutions, registries, and research studies to create larger, more comprehensive datasets, increasing statistical power and enabling more robust analyses. The ability to capture and use RWD can complement and enhance clinical research data. For example: Aggregation of datasets available from sponsors and those obtained through patient registries and public data databases.
2. Standardization and harmonization
Provides metadata and standards management capabilities and implements standardized data formats and collection protocols to ensure consistency and comparability across different sources and facilitate effective integration and analysis . For example: Ability to uphold research standards defined by the sponsor/CRO and industry standards such as SDTM, CDASH, and OMOP.
3. Data validation and curation
It serves as a data management workbench for personnel to focus on data validation and cleanup. This increases data accuracy and completeness, minimizes bias, and increases reliability. Example: A facility reviews her CRF data collected from a patient to ensure data quality.
4. Completion of missing data
It provides statistical computing capabilities within the platform and enables the adoption of statistical methods to address missing data gaps, further enriching datasets and enabling more comprehensive analyses. For example: The availability of a statistical computing environment at UCDR is useful for advanced statistical analysis and data engineering using machine learning models to impute data.
5. Secure data platform
This allows users to securely store and share data through a controlled-access platform, enabling collaboration and knowledge exchange while addressing privacy concerns. For example: Providing patient registration data or other anonymized data for third party research in a secure manner, ensuring that only the intended parties can access it for the intended purpose.
6. Common data model and interoperability tools
Break down technical barriers with common data models and interoperability tools to enable seamless data exchange and analysis across different platforms and systems. For example: Using data models that are based on industry standards such as SDTM or that support data exchange standards such as HL7 FHIR.
7. Transparent data governance
We have a robust data governance structure and transparent policies that promote responsible data sharing while adhering to ethical principles and protecting patient privacy. For example: Ensuring that the origin of the data collected is properly maintained, tracked and available for audit and inspection purposes if necessary.
8. Advanced data analysis
Integrated clinical data cloud platforms with modern architectures like Data Lake House (a new architecture that combines the best elements of data warehouses and data lakes) enable researchers to apply advanced analytical techniques such as ML. , you can identify patterns and insights hidden in vast amounts of data. Set (enhanced by RWD availability). Examples: Using statistical models to avoid identifying outliers in collected data, or using machine learning models to drive data standardization and anomaly detection.
9. Collaborative research environment
By streamlining data access and sharing, cloud platforms foster collaboration between researchers, institutions, and industry, accelerating progress in rare disease research. Example: An integrated clinical cloud platform can serve as a central repository that can provide access to internal users for data management, biostatistics, and statistical programming, and make data available to external academic researchers and others as needed. You can also.
An integrated clinical data cloud platform for rare diseases fundamentally addresses data challenges and paves the way for rapid advances in diagnosis, treatment, and ultimately improved quality of life for individuals living with rare diseases. has proven to be a powerful tool.
About the author:

Venu Mallarapu is a business and technology leader with over 25 years of industry experience. He has supported organizations with business and his IT advice, strategic consulting, relationships, and delivery management. As vice president of global strategy and operations for eClinical Solutions, Venu drives overall strategy and operational excellence to drive adoption, market expansion, and utilization of the elluminate® platform and services.
Venu is a small company responsible for all research and development functions. He has provided strategy and transformation advice to the world’s top 50 life sciences companies. He regularly speaks at events about transformation, innovation, and next-generation technology. He publishes articles, blog posts, webinars, and seminars on topics that advance the industry and is recognized as an industry thought leader.
[ad_2]
Source link


