research design
This study was a single-blind, randomized controlled trial. This study was approved by the Ethics Committee of the Second Affiliated Hospital of Guizhou University of Traditional Chinese Medicine (KYW2022007). This study complied with the provisions of the Declaration of Helsinki and was conducted from April 2022 to March 2023 at the cardiac ward of the Second Affiliated Hospital of Guizhou University of Traditional Chinese Medicine, Guiyang, China. Eligible participants were randomly assigned to one of the interventions. Group using sequentially numbered sealed opaque envelopes or control group. All randomization procedures were performed by investigators who were not involved in patient recruitment, exercise training, or outcome assessment. Because participants were recruited continuously over the course of the study rather than at a single time point, a permuted block randomization technique was used to ensure equal sample size between groups over time.twenty one. If 8 participants were successfully recruited, a randomization procedure (i.e., block size = 8) was performed with an allocation ratio of 1:1 (i.e., 4 participants were assigned to the intervention group). , four were assigned to the control group). Outcome variables were measured at baseline and 3 months after the intervention. A single-blind design was maintained as the research assistants performing data collection did not have access to information regarding group assignment.
sample size
Sample size was estimated using the following formula for two-group comparison with random design:
$$\mathrm{NI}=\mathrm{N}2=2\left[\frac{({\mathrm{Z}}_{\mathrm{\alpha }}+{\mathrm{Z}}_{\upbeta })\mathrm{ S}}{\updelta }\right]2$$
here Zα: Z value corresponding to type I error α. Zβ: Z value corresponding to type II error β. \(\mathrm{S}\): standard deviation; and \(\UpDelta\):allowable error.
A one-sided test was employed with α = 0.05, β = 0.20, and Z.α= 1.96, and Zβ= 1.06. Total cholesterol, triglycerides, and low-density lipoprotein cholesterol were the primary outcome measures. \(\mathrm{S}=\) 0.26 and \(\UpDelta\)=0.18 Based on review of related literaturetwenty two. Therefore, the calculation result is N1 = N2 = 20. Assuming a 20% attrition rate, she needed to recruit 24 patients into each group.
participant
During the study period, patients received medication and PCI in the hospital for AMI, and were regularly monitored clinically after discharge. Participants were recruited through study promotional posters and referrals from hospital staff. Researchers examined participants’ medical records and conducted interviews to determine eligibility for individuals who expressed interest in participating. Participation criteria: 60-75 years old. Ability to use electronic equipment. Unimpaired physical activity and ability to engage in self-care. Left ventricular ejection fraction ≥40%. A signed informed consent will be provided. Must be willing to participate in a complete program of study without assistance. Patients were ineligible if they met any of the following exclusion criteria at the time of screening: Institutional investigators determined that the patient was unable to complete the study and/or attend the follow-up visit. Regular practice of Ba Duan Jin, i.e. 3 or more sessions per week. Concurrent participation in other clinical trials. and concomitant other medical conditions or malignancies (e.g., severe valvular disease, New York Heart Association Class IV, heart failure, severe aortic regurgitation, cancer, and end-stage renal or liver disease).
intervention
Usual treatment and care
After discharge, patients received regular medical and nursing care from community-based physicians and cardiologists. Conventional treatment consisted of postoperative administration of 100 mg aspirin (Bayer Leverkusen, Germany) once a day for long-term maintenance. He was prescribed oral ticagrelor (AstraZeneca, Wilmington, DE) 90 mg twice daily for 1 year. Other drugs (statins, angiotensin-converting enzyme inhibitors, angiotensin II receptor antagonists, calcium channel antagonists, and β-receptor antagonists) were administered as needed, depending on the patient’s condition. Routine care consisted of her post-PCI patient health education and medication management information provided by the ward nurse before discharge, and regular follow-up. Daily medical treatment was based on the 2018 simplified version of the Chinese Cardiovascular Rehabilitation and Secondary Prevention Guidelines.twenty three.
control group
Patients assigned to the control group received traditional in-hospital CR training combined with Baduanxing, a traditional Chinese gymnastics exercise. Specific rehabilitation training is as follows. 10 minute breathing training. 15 minutes of aerobic exercise. A resistance exercise consisting of 10 minutes of simple dumbbell exercises. He performs the Ba Duan Jin exercise for 10 minutes (see appendix for specific Ba Duan Jin movements, repeating each of the 8 segments 3 times). 10 minute balance and flexibility exercise relaxation training. Patients were encouraged to visit the hospital three times a week and do 50 to 60 minutes of exercise each time. The age-predicted target heart rate (220 minus the patient’s age) was used as a measure of intensity for the aerobic portion of the exercise training plan.twenty four. For all participants, the target chosen for the aerobic training portion of the program was his 60% of his age-predicted target heart rate.General assessment of perceived efforttwenty five It was also used to assess exercise intensity, using a scale of 11 to 14 as an exercise intensity index.
intervention group
In addition to the exercise protocol, the intervention group remotely managed CR using wearable smart devices and a 5G IOT CR intelligence platform. CR was performed three times per week for 50–60 minutes each session. The total number of interventions was 36 sessions.
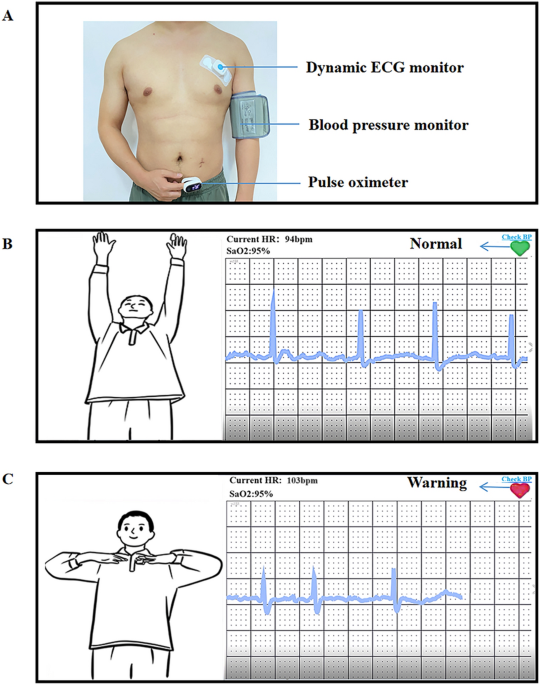
Researchers tested whether patients’ mobile phones were 5G compatible. If not, the researchers provided patients with 5G-enabled mobile phones and installed the Healthy Life Cycle application (app) before patients were discharged from the hospital. Patients also received a wearable smart remote monitoring device connected to the Healthy Life Cycle App via Bluetooth (pulse oximeter, model: A0J-70C, Shenzhen, China; dynamic electrocardiogram (ECG) monitor, model: ECG -P01, Hangzhou, China, arm-type electronic blood pressure monitor, model: B65T, Shenzhen, China) (Figures 1, 2).

Portable cardiac rehabilitation device.

Operation of 5G Internet of Things cardiac rehabilitation platform.
With the help of researchers, patients were asked to log into the Healthy Life Cycle app, click on “Rehabilitation Center” to access the online program, wear a wearable device to monitor their vital signs, and upload their data to the app. (Figure 3). ). The online program was held twice a day during her weekdays at 9:00 a.m. and 2:30 p.m., and patients could choose which session they would like to attend.
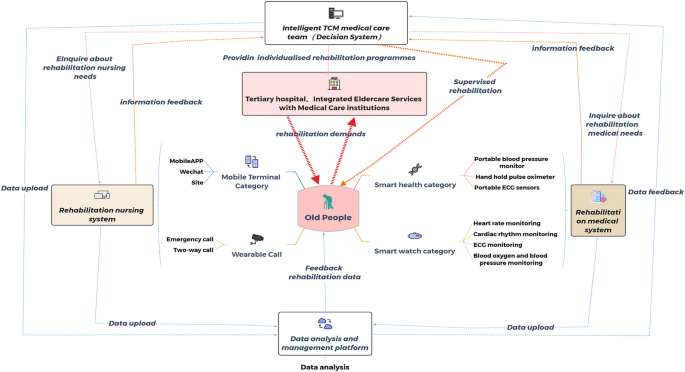
5G + IoT smart CR service architecture.
Rehabilitation exercise classes were the same as the control group. Vital signs were recorded after each exercise, and the researchers verified that there were no abnormalities before the patients moved on to the next part of the exercise program. Patients can monitor their own training and vital signs through the app, and rehabilitation staff can adjust the patient’s exercise training pattern, intensity, and time based on changes in vital signs during exercise, providing rehabilitation tailored to the individual. realizable.
A psychologist was assigned to this study and provided psychological counseling once a month to ensure the psychological well-being of the patients.
result
Primary outcomes included changes in cardiorespiratory fitness, physiological indicators, and psychological indicators. Secondary outcomes were patient exercise compliance, adverse cardiovascular events, and patient satisfaction with rehabilitation care during the intervention. Cardiopulmonary exercise capacity, physiological indicators, and psychological indicators were measured before the intervention (baseline) and 3 months after the intervention. Patients’ exercise compliance during the intervention, adverse cardiovascular events, and satisfaction with rehabilitation care were investigated 3 months after the intervention.
Cardiorespiratory capacity was measured using a Cyclagometer cardiopulmonary exercise testing device (CS-200 Ergo-Spiro, Schiller, Switzerland) according to relevant cardiopulmonary exercise testing standards.26. Maximal oxygen uptake (VO)2max) and task metabolic equivalent (MET) were used to assess patients’ cardiac functional status and exercise capacity, respectively. Physiological indicators include high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, triglycerides, total cholesterol, and BMI. Psychological indicators were assessed using the Patient Health Questionnaire-9 (PHQ-9) scale.27 and Generalized Anxiety Disorder-7 (GAD-7) scale.28.
Compliance with rehabilitation exercises and satisfaction with rehabilitation care were assessed by self-administered questionnaires. MACE, i.e. recurrent myocardial infarction, malignant arrhythmia, heart failure, and angina pectoris, were recorded in both groups during the intervention.
statistical analysis
Statistical analyzes were performed using SPSS version 25.0 (IBM Corp., Armonk, NY). Data were reported as mean ± SD for continuous variables and as number and percentage for categorical variables. Two independent samples t-tests were used for comparisons between groups. Analysis of covariance models was used to estimate least squares (LS) mean differences by treatment (intervention vs. control) and baseline values of variables assessed as covariates. The 3-month LS mean and between-group differences in LS mean were calculated for each variable along with the standard error, 95% confidence interval, and 95% confidence interval. p values. χ2 The test was used to compare categorical variables between groups.All reported p The value is two-sided; p<0.05 was considered to indicate statistical significance.